Nestlé issued an unprecedented global recall for several infant formula lines, including SMA, BEBA, and NAN on Monday, 5 January 2026.
The move followed the detection of cereulide, a potent toxin produced by Bacillus cereus bacteria, in a key ingredient, arachidonic acid (ARA) oil, provided by a third-party supplier.
Spanning more than 25 countries and impacting over 800 products from 10 different factories, this is reportedly the largest recall in Nestlé’s history. While no illnesses have been confirmed, the company has urged parents to stop using affected batches immediately and seek refunds.
Understanding Cereulide
The recall was triggered by a quality issue at a supplier’s facility, where a “technical cleaning defect” led to the contamination of oil mixes used across multiple production lines in late 2025.
Cereulide is a particularly dangerous toxin because it is highly heat-stable, meaning standard preparation methods like using boiling water or heating the formula do not deactivate it.
If consumed, it can cause rapid food poisoning symptoms, including severe nausea and vomiting, within 30 minutes to six hours. This underscores the severity of the alert, as traditional safety measures used by parents in the kitchen are ineffective against this specific contamination.
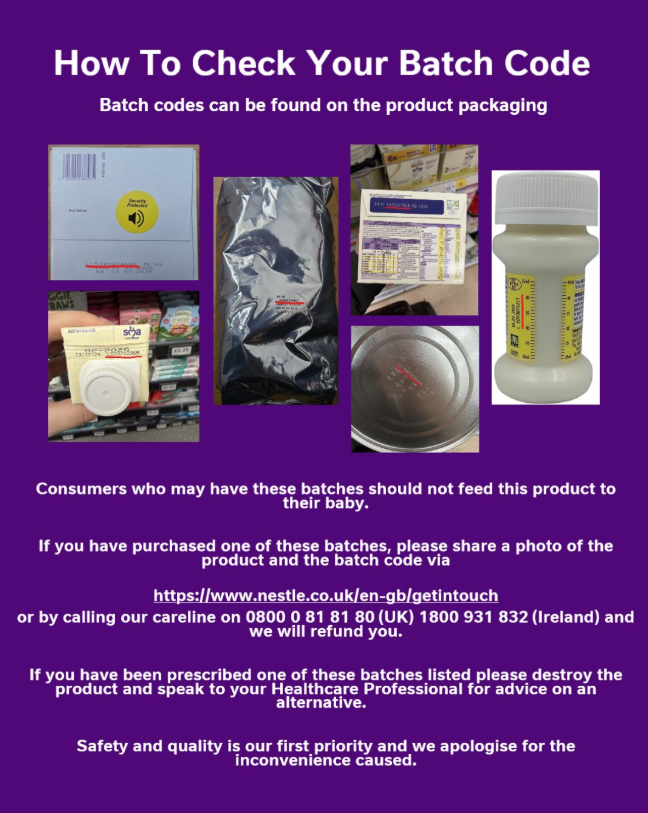
As a precautionary measure, Nestlé is voluntarily recalling specific batches of its SMA infant formula and follow-on formula. This is due to the potential presence of cereulide in the batches concerned. A full list of products and batch numbers can be found here on the images… pic.twitter.com/Qwbt7WsEHI
— Nestlé UK & Ireland (@NestleUKI) January 5, 2026
Nestlé Recall
While the recall is most concentrated in Europe, affecting nations such as the UK, Ireland, Germany, France, and Switzerland, it has extended to markets as far as Argentina, Turkey, and Saudi Arabia.
Nestlé has published comprehensive lists of batch codes found on the base of tins or ready-to-feed containers to help caregivers identify affected stock.
“The safety and wellbeing of babies is our absolute priority,” a Nestlé spokesperson stated, emphasizing that the recall is a “precautionary measure” taken in full alignment with local health authorities.
Agencies like the UK’s Food Standards Agency (FSA) have reinforced this, advising that even if a child seems healthy, affected formula must be destroyed immediately.
The Logical Indian’s Perspective
At The Logical Indian, we believe that when the safety of infants is at stake, the margin for error must be zero. While Nestlé’s voluntary and transparent recall is a necessary step, the sheer scale of this incident, impacting over 800 products, exposes a fragile global supply chain where a single cleaning defect can jeopardize children across continents.
We advocate for a future where corporate accountability includes more rigorous, real-time testing of third-party ingredients before they reach production lines. Profit and growth must never outpace the fundamental promise of safety. We urge all parents to remain vigilant and prioritize medical advice over convenience.












