The DCGI (Drug Controller General of India) approved a potential COVID-19 vaccine, the first to be developed in India, COVAXIN, which is set for Phase I and Phase II human clinical trials. COVAXIN has been developed by Hyderabad-based Bharat Biotech, in association with ICMR (Indian Council for Medical Research).
Four days after India’s first indigenous COVID-19 vaccine candidate got approval. ICMR on Thursday, July 2, announced that it wanted to ‘launch the vaccine for public health use latest by August 15’. The ICMR set the ambitious launch date in partnership with Hyderabad-based Bharat Biotech International Limited (BBIL).
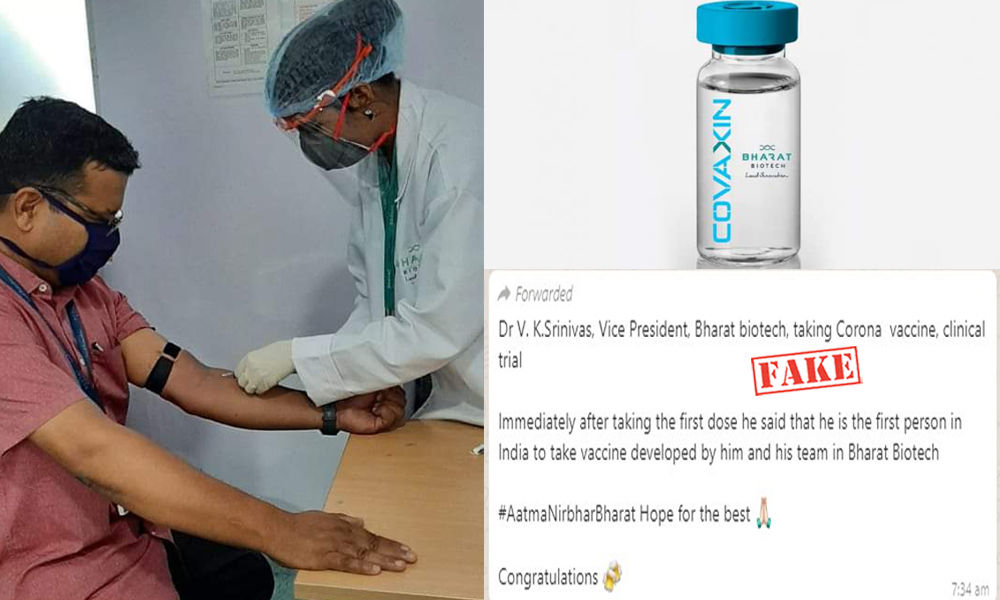
In light of this, a piece of news is doing the rounds on social media which said that the first dose of the vaccine was administered on BBIL Vice President VK Srinivas as part of the human trial.
Below is the viral photo:
The picture that is being circulated shows a woman in medical gear thrusting a syringe in a man’s veins.
‘Dr VK Srinivas, Vice President, Bharat biotech, taking Corona vaccine… Clinical trial immediately after taking the first dose he said that he is the first person in India to take vaccine developed by him and his team in Bharat Biotech. Look at the confidence that they have in their product,’ reads the caption of the viral posts that are doing the rounds.
Dr VK srinivas vice president Bharat biotech taking coronavirus vaccine clinical trial he is the first person in India to take vaccine developed by him and his team in Bharat biotech. pic.twitter.com/bz5C88f9ot
— jaggirmRanbir (@jaggirm) July 4, 2020
We know that Bharat Biotech has already on human trial of Corona Vaccine. Dr V K Srinivas ,Vice President Bharat Biotech has taken First Corona Vaccine clinical trial and he said he is first person in India to take this Vaccine develop by him and his team.We Salute pic.twitter.com/5waK4KGILC
— Dr JB Tiwari (@drjbtiwari) July 4, 2020
The claims are also viral on Facebook.
The Logical Indian received a request to verify the claims made about the viral photo.
Claim:
The first dose of COVAXIN was administered on Bharat Biotech Vice president VK Srinivas as part of human trial.
Fact Check:
The claim is false.
Bharat Biotech Refutes Claim
After the photograph started doing the rounds, the company took to Twitter to debunk the claims.
A statement issued by BBIL said, ‘Certain images and messages being circulated on WhatsApp and other social media platforms have NOT been disseminated by Bharat Biotech. The image being circulated is a routine procedural blood draw for testing all production staff.’
pic.twitter.com/m1JCA5rWmW
— BharatBiotech (@BharatBiotech) July 3, 2020
Further, On July 1, Dr Krishna Ella, chairman and MD of Bharat Biotech, had said in an interview with NDTV that ‘human trials should start in 10 days’.
#LeftRightCentre | ‘Most of the clearances are coming through and in 10 days human trials should start’: Dr Krishna Ella, Chairman and MD of Bharat Biotech on COVAXIN, India’s first vaccine candidate for #COVID19 pic.twitter.com/GqaOUN1NEQ
— NDTV (@ndtv) July 1, 2020
Therefore, human trials must not have started yet.
VK Srinivas Is The Vice President For Bharat Biotech?
On searching for the ‘Vice President’, VK Srinivas in the Bharat Biotech website, no results could be found.
However, the Executive Director and Senior Vice President of BBIL is V Krishna Mohan and the Chief Financial Officer in the Management team is T Srinivas.
If you have any news that you believe needs to be fact-checked, please email us at factcheck@thelogicalindian.com or WhatsApp at 6364000343.
Also Read: ICMR Asks To Fast-Track COVID Vaccine, Experts Call It ‘Unrealistic’