The Indian Council of Medical Research (ICMR) on July 2 had announced that it wanted to ‘launch the vaccine for public health use latest by August 15’. The ICMR had set the ambitious launch date in partnership with Hyderabad-based Bharat Biotech. Bharat Biotech is the maker of the world’s cheapest Hepatitis vaccine.
As the date of the apparent ‘launch’ of the vaccine approaches, WhatsApp Forwards, social media posts claim that it will be available in the market from 15 August.
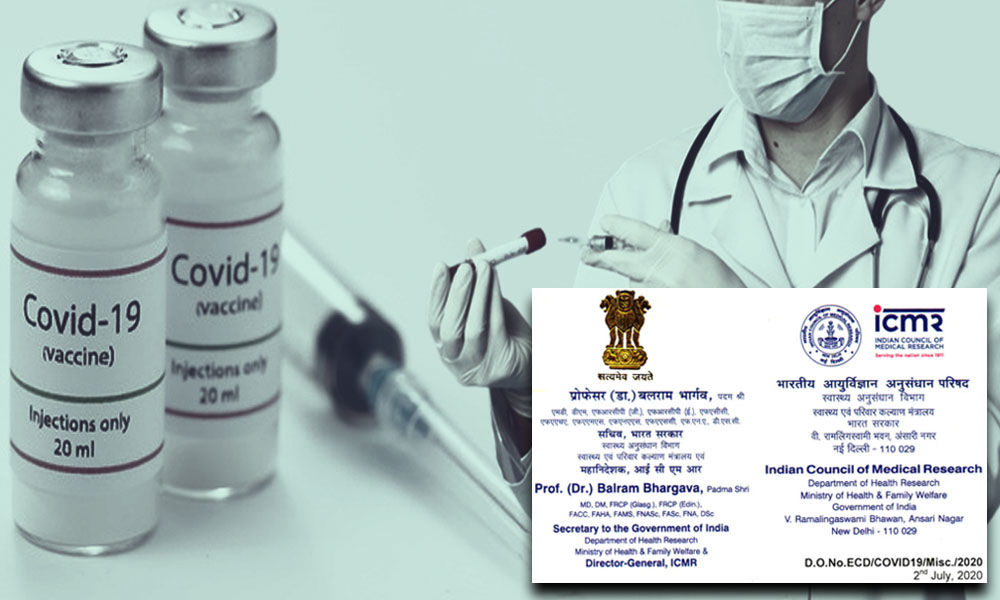
A letter from ICMR about fast-tracking the clinical trials of the vaccine is also doing the rounds.
‘Bharat biotech vaccine Received permission from central government from 15th August it will be available in market as corona vaccine . Great news today see official permission,’ reads the WhatsApp forward.
The Logical Indian received multiple requests asking if the vaccine will be developed by Bharat Biotech by August 15. The claim was shared on Twitter and Facebook also.
Bharat Bio Tech Vaccine Received Permission From Central Government From 15th August It Will Be Available In Market As Corona Vaccine . Great News Today See Official Permission pic.twitter.com/Khzi78ajxT
— YS JAGAN 4 VIZAG (@NaniYsrcpYzag) July 21, 2020
Claim:
Bharat Biotech’s COVID-19 vaccine will be available in the market from 15 August, according to a letter from ICMR about fast-tracking the clinical trials of the vaccine.
Fact Check:
The claim is false. The message is real, however, there have been clarifications about the letter. The clarification says that 15 August is not actually a deadline for the launch of the vaccine.
ICMR’S Push To Fast-Track Process
On July 2, the apex medical research body’s director-general Balram Bhargava wrote to a dozen institutions involved in the trial to ‘fast-track all approvals’, adding that participants for the trial should be enrolled ‘no later than July 7’.
Bhargava warned that non-compliance will be viewed very seriously.
‘Therefore, you are advised to treat this project on highest priority and meet the given timelines without a lapse,’ he wrote in the letter.
The letter revealed a plan to launch India’s COVID-19 vaccine ‘COVAXIN’ developed in collaboration with Hyderabad-based Bharat Biotech Limited (BBIL) by August 15.
However, it also stated that ‘final outcome will depend on the cooperation of all clinical trial sites involved in this project.’
This point is not mentioned in the viral claims.
‘The vaccine is derived from a strain of SARS-CoV-2 isolated by ICMR-National Institute of virology. Pune- ICMR and BBIL are jointly working for the preclinical as well as clinical development of this vaccine,’ the letter further read.
The medical body had asked 12 institutes to fasten up their clinical trials of the vaccines, to enable the subject enrolments by the first week of July.
‘You are strictly advised to fast track all approvals related to the initiation of the clinical trial and ensure that the subject enrolment is initiated no later than July 7, 2020. And non-compliance will be viewed very seriously. Therefore, advised to treat this project on the highest priority and meet the given timelines without any lapse,’ the letter read.
ICMR Clarification
After an outrage by medical community stating that how can a vaccine be developed in such a short time and ICMR threatening the scientists working on the vaccine. On July 4, in an official statement, ICMR clarified that its process was ‘exactly in accordance with the globally accepted norms to fast-track the vaccine development for diseases of pandemic potential wherein human and animal trials can continue in parallel.’
‘Our internal communication is being misinterpreted. We only said that we envisage to have a vaccine by 15 August and it is not a deadline. We have not said that we will launch a vaccine by then. The process can be expedited but the vaccine still will have to undergo all safety clinical trials,’ LiveMint quoted an ICMR official as saying.
According to the apex body, it aimed at completing the trials quickly, similar to vaccine candidates in other countries, considering the urgency of the ongoing pandemic.
‘Just as red tape was not allowed to become a hindrance in the fast-track approval of new indigenous testing kits or for introducing in the Indian market potential covid-19 related drugs, the indigenous vaccine development process has also been sought to be insulated from slow file movement,’ an ICMR statement said.
Bharat Biotech’s Stance
On being asked when can the vaccine be expected, Dr Krishna Ella, Bharat Biotech founder told The Week in a report dated July 26 that ‘It is too early to remark on this, as we are in the beginning phase of human trials. Only after the safety data is established and upon receiving regulatory approvals will we be able to decide to move into the course of licensure.’
Further, In an interview with New Indian Express, Dr Ella had said that ‘if the clinical trials of Covaxin go well and meet the highest safety and efficacy standards, and if…