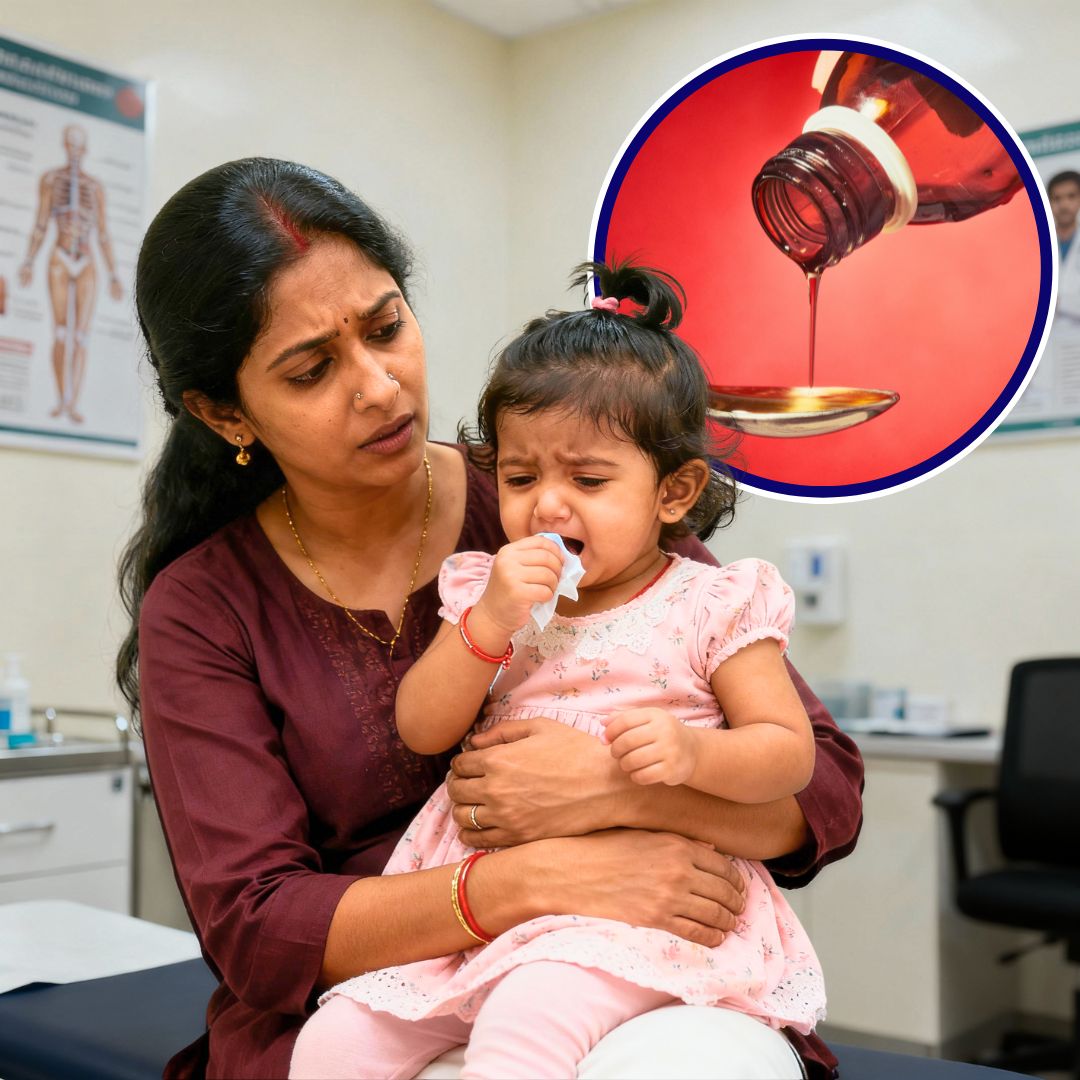
Following the deaths of infants linked to contaminated cough syrups, the Indian Pharmacists Association has directed all pharmacists not to dispense any cough or cold preparations to children under two years, even with a prescription, the economic times reported.
The advisory, issued in line with Union Health Ministry and CDSCO guidelines, warns of life-threatening risks like respiratory depression and toxicity.
The directive follows a high-level meeting chaired by Union Health Secretary Punya Salila Srivastava on October 5, 2025, and comes amid investigations into toxic batches of Coldrif syrup in Madhya Pradesh and Rajasthan. Pharmacists are now required to counsel caregivers and suggest non-drug alternatives like hydration and saline drops.
Pharmacists Given Clear Directive to Withhold Medications
The Indian Pharmacists Association has issued a strict advisory stating: “All Pharmacists working in community and hospital pharmacies are hereby advised to strictly comply with the guidelines issued by the Ministry of Health & Family Welfare (MOHFW) and the Central Drugs Standard Control Organisation (CDSCO) regarding the non-use of cough and cold preparations in children below two (2) years of age”.
If a doctor prescribes such medication, pharmacists must inform the prescriber and recommend a review in line with national guidelines. The advisory highlights that these syrups can cause “serious and potentially life-threatening adverse effects in infants, such as respiratory depression, excessive sedation, and toxicity”.
Pharmacists are also instructed to educate parents on safer alternatives, including hydration, saline nasal drops, humid air, and paediatric consultation.
National Response to Prevent Further Tragedies
The advisory follows a high-level meeting convened by the Union Health Ministry on October 5, 2025, where states and union territories were urged to enforce rational use of cough syrups and ensure compliance with revised Schedule M norms for drug manufacturing.
Union Health Minister JP Nadda had earlier directed a review of the issue with state authorities to prevent future incidents. The crisis was triggered by the deaths of at least 20 children in Madhya Pradesh and Rajasthan after consuming contaminated Coldrif syrup containing toxic diethylene glycol (DEG).
Despite a 2023 CDSCO ban on certain ingredients in paediatric formulations, poor enforcement and outdated labelling allowed dangerous products to remain on shelves.
The Logical Indian’s Perspective
Every child’s life is precious, and no parent should fear that medicine meant to heal could cause harm.
The pharmacists’ advisory is a vital step toward safer care, placing ethical responsibility directly in the hands of those dispensing medicine. But true protection requires more, stricter manufacturing oversight, transparent labelling, and public awareness.